Often smell of molecule!
2005/04/01 Odriozola Agirre, Ibon Iturria: Elhuyar aldizkaria

The smell is undoubtedly the most sensitive sense and is special, since the olfactory epithelium is formed by simple nervous terminations. This does not happen with other sensations (except pain), since normally a transducer acts as a buffer between the outside world and the nervous system.
Through smell, the nervous system is directly related to the outside world. Basically, the brain is visible in the nose, which indicates that smell is the oldest and most primitive of the senses. The smell is also closely related to the physical closeness of its processing centers with at least one of the most primitive parts of the brain: the limbic system, the center of control of emotions. This can explain the strong impact of smell and sometimes the cause of the unconscious.
In the following lines we will talk about the chemistry of sensation. All sensations are, in short, chemical, since all the neuronal activity of our brain is based on the transport of molecules and ions and on the reactions in which they intervene. Some messages that living beings receive from the outside world depend on the perception of certain molecules that act as messenger. Smell is an example of chemorescence.


Here I do not want to explain the complex physiology of smell, nor the relationship between molecular structure and smell. The aim of this study is to analyze which compounds produce odors known to all.
When the aromatic molecules evaporate in the air, they reach the marrow of our nostrils and dissolve in it. Then, olfactory nerves send nerve impulses to the brain, which causes a sometimes positive and sometimes negative feeling.
We live surrounded by molecules and should not fear. We breathe, eat, drink, smell, excrete or garment molecules, and yet we only know a few. Some are very complex structurally and others very simple, but their influence on beings is not at all related to the complexity of the structure.
A molecule: a molecule

All the material around the world, whether it's a piece of rock, a tree, a chicken or a Rolex, is made up of a tissue of simple substances called chemical elements. Among them are hydrogen, carbon, oxygen, or copper.
The smallest part of a possible element is the atom (of the Greek, atomos, incortable). Part of a pure element, like a piece of gold, is only a set of atoms. The atoms are very small. The diameter of a carbon atom is 0.0000000000015 meters (1,5 x 10 -10 m), so a carbon line of 1,5 cm of approximate length is: ) is one hundred million atoms of limestone from the edge to the edge, and one million atoms or indirect. In the smallest dust we can see there are more atoms in our galaxy than stars.
Many compounds are formed by molecules. The molecule is a specific combination of two or more atoms that bind together in a certain geometric arrangement. Although in nature there are more than one hundred atoms, all the molecules described below are combinations of up to five atoms of hydrogen, carbon, nitrogen, oxygen and sulfur. Let's see now the aspect that usually have the molecules that introduce us in our nostrils and that produce sensations.
Good smells...

A smell can produce memories, sometimes positive and sometimes negative. Many people have memories of their childhood, for example, smelling phenol (C 6 H 6 O), “because it smells like school paintings.”
When we talk about pleasant odors, many times the perfumes come to our heads. In fact, perfumes are made for that, to fascinate people with the pleasure of smell.
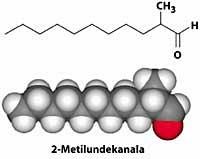
2-The metatardean (C 12 H 24 O) is the main molecule of the Channel N> 5 fragrance, presented by Coco Channel in 1921. With this perfume, the perfume industry began to use synthetic compounds for the first time for perfumes to reach everyone.
The chemicals dedicated to perfumery use a unique language to describe their achievements: "Paco Rabanne pour home is born to remember a summer walk with fresh air through the hills of Provence; herbaceous odors, rosemary and thyme; and brilliant freshness mixing with the cold sea wind and the warm air of the Alps. To achieve the necessary effect, the perfumer has mixed the grass oils with touches of wood and the chemical aroma of dimethylheptanol, so that its penetrating but indefinable freshness recalls the outside air or the freshly washed flax". (J. Ayres, Chemistry and Industry , 1988, 579).
The fragrance content of perfumes is reduced to 5-10%, while the rest is usually an ethanol/water mix (90:10). Therefore, in the perfume industry a lot of ethanol is required and, as can be thought, not so much of aromatic compounds. The annual production of several important fragrances, such as jasmine, is usually around 10,000 tons. And pure perfumery ingredients like Cis -Jasmona (C 11 H 16O, main component of jasmine) cost hundreds of euros per gram.

Damascus (C 13 H 18 O) is the main responsible for the smell of pink. However, if we open the bottle and put the nose, we will take a hard disenchantment, since it has a smell similar to the camper or turpentine. But the next morning, we and the clothes that dressed us will give us a smell of pink that develops with dilution.
In essential vegetable oils abound the aromatic compounds of the family of terpenes, which cause almost all pleasant sensations such as camphor (C 10 H 16 O), menthol (C 10 H 20 O) and geraniole (C 10 H 18 O). The first two, well known, are used in Reflex or Vicks VapoRub products, generating in general a pleasant feeling of freshness. The second, as can be deduced from the name, is found in the geranium and participates in its smell.
...and bad smells
Our smell of sweat is largely due to short and medium chain carboxylic acids (compounds with COOH group).
The acid formed by four carbon atoms, CH 3 (CH 2) 2 COOH, is the main responsible for the smell of mined butter and is known as butyric acid (butirum, latin, butter). This compound is very common in vomiting and sweating of the feet. We do not mistake the smell of sweat of the feet when we call it smell of cheese, since in some cheeses of strong smell there is that same compound. According to an American study, the perception of the smell of this compound varies greatly according to the context. Normally, the smell of butyric causes an unpleasant reaction in people, but if they are told to think about food, the smell becomes more pleasant.

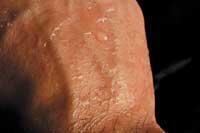
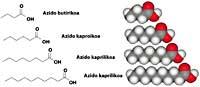
Caprylic, caprylic and caprine acids have 6, 8 and 10 carbon atoms respectively, and are oily liquids with a goat smell (caper, Latin, goat). In addition to goats, people secrete these compounds (more than others) and are part of the smell of our armpit. Dogs can know the sweat smell of their owner by its content in carboxylic acid.
Every year, men and women spend large amounts of money on soaps, gels and deodorants that avoid these odors, since the smell of goat is unaesthetic and antisocial for us. It is interesting that the same malolient compounds we spend even more to reproduce in our body, since caproylic, caprylic and caprylic acid are found in most essential perfumes and oils.
Ammonia, NH 3, is a colorless and flammable spicy gas under normal conditions. Very soluble in water, it influences its smell as it is easily dissolved in the aqueous mucosa that covers the nasal olfactory epithelium. The smell of water would probably be as spicy as ammonia if our sense organs were not so accustomed to it.
Camembert or wet Brie also smell ammonia, a gas that forms as nitrogen proteins break down. We should thank the microbes for the smell that usually exists in the cantons of the streets of our villages on Sundays in the morning, since with their work, the urea that people have left there the eve with the best will, that is, the main component of the urine, becomes ammonia.
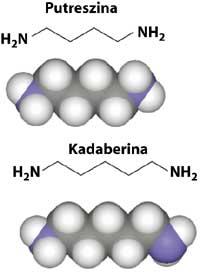
The smell of rotten meat reminds us of amines, organic derivatives of ammonia. Amines preserve the granularity of ammonia, but often in a more transformed and unpleasant way. In the case of putrescine (C 4 H 12 N 2) and cadabine (C 5 H 14 N 2), the name itself gives us a pretty clear idea of its smell. In addition to being the main responsible for the smell of rotten meat, they participate in a lower proportion in the smells of semen and urine, as well as in the breathing.
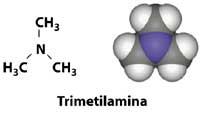
The next time we smell rotten fish, we should think of trimethylamine, the molecule that we will have inside the nose. This compound is derived from the enzymatic degradation of fish proteins. House dogs also sometimes have a smell of fish, since they can be detached from this amine. The disease called trimethlaminurea, more frequent in women than in men, is due to a defective enzyme that causes the degradation of proteins and that secretes this compound of smell of fish in sweat.
In view of all these examples, it seems that we know well what molecules produce the signal that reaches our brain every time we smell a flower or a freshly made coffee. Now, unfortunately, we are still far from understanding what happens in our mind, how our emotions respond, or what our pleasure consists of, even though the advances of science are increasingly exciting.


Sulfur, king of bad odors
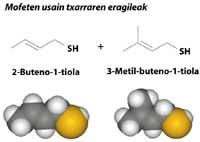

As for bad odors, many of the sulfur derivatives are not left behind. For example, the smell of the spray that secretes the mofet is a mixture of two thiols, sulphur derivatives with SH group.
But, it seems, the worst of all odors is what caused the evacuation of the city of Freiburg in 1889, according to those present. During the conduct of a trial to obtain tioacetone through the cracking of tritioacetone, an "offensive smell that was quickly distributed to a large part of the town" was constituted, which caused dizziness, vomiting and evacuation to coal. Of course, laboratory work was suspended.
Workers at an Esso research laboratory, perhaps with great courage, repeat the same experiment south of Oxford in 1967. Although the cap of a bottle of waste leaped by accident and closed immediately, it did not take long until the members who were working in the other building, located about two hundred meters away, complained about the muddle and discomfort.
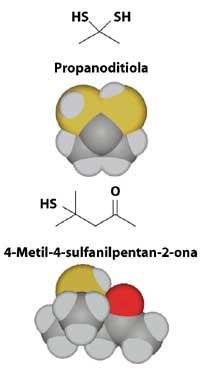
Only one of these two compounds seems to be responsible for this unbearable smell: propanoditiola or 4-methyl-4-sulphylpentan-2-ona. Surely it will be a long time until someone has the courage to resolve that debate.
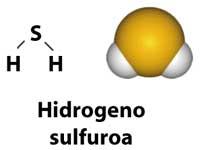
Hydrogen sulfide, H 2 S, can be considered as a water-like sulfur. In the center it has an atom of sulfur instead of oxygen. However, this simple substitution carries notable differences, do not think so.
Hydrogen sulfide is a poisonous gas with irritating odors. The characteristic smell of rotten eggs is due to it and, in less concentration, to the pleasant smell of freshly cooked eggs.

Whoever has ever eaten Aza will already know (both he and others) that the smell of hydrogen sulfide is no joke. The cabbage leaves are rich in amino acids with sulfur, and when broken down by bacteria from the large intestine, gas H2S is released, among others. That, of course, comes from behind.
Annoying odors have their uses. The gas that reaches our homes through the tube is added sulphur derivatives like ter-butiltiol (CH3)3CSH at low concentrations, since the gas itself has no smell and otherwise we could not detect gas leaks. By low concentration, it is understood to be really low; humans can detect a part of 50,000,000,000 natural gases.
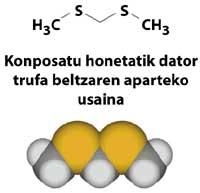
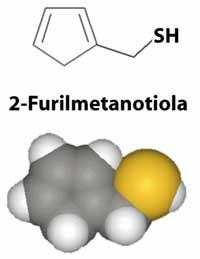
However, do not think that all sulfur derivatives have disgusting odors. To dishonor the honor of these compounds, we must mention the precious smell of truffles that pigs can find under the ground at a meter of depth, which for its excellent smell and flavor is sold more expensive than gold. The pleasant smell of freshly made coffee is also due to a sulfur derivative, 2-furilmetanotiol (C 5 H 6 OS).
More information More information
- Atkins, P. Atkins' Molecules Cambridge University Press Cambridge, UK. 2003. Clayden, J., N. N. Greeves, Warren S. and Wothers, P. Organic Chemistry Oxford University Press, Oxford, UK. 2001. Meierhenrich, U. J. Golebiowski, J., Fernández, X., and Cabrol-Bass, D. Angew Chem. Int. Ed. 43, 6410-6412. 2004 Chemical Engineering News October 11, 11. 2004 2004