Vanadium as a steel motor
2002/03/01 Rodriguez Ibabe, Jose Maria | Florez Esnal, M.A. Iturria: Elhuyar aldizkaria
Incidences of the discovery

The Vanadium was first communicated in 1801. Andrés Manuel del Río discovered vanadium in the lead mines of Zimapa, in Mexico, which then depended on Spain. This Madrid geologist and chemist was appointed professor of the “Real Seminario de Minas” directed by Fausto Elhuyar in Mexico in 1795 and, together with Fausto, began to improve the exploitation of Mexican mines. Thus, Andrés Manuel del Río discovered that in the mineral lead extracted from the mines of Zimapán there was another element until then unknown. At first he called this new element ‘panchrome’, but later, seeing that when heated he acquired a reddish color, he called it ‘eritronio’.
A year later, the River gave his friend Alexander von Humboldt some samples of which Humboldt sent Collet-Desotils to the prestigious French chemist of the time. Collet-Desotils, who taught in Paris, confirmed his own investigation of the elements of chromium and iridium discovered years earlier. Therefore, his opinion was valuable. Collet-Desotils questioned Rio's work, arguing that the new element was nothing more than a lead chromate. It seems that Del Río also had doubts and forgot the vicissitudes of the new element.
In 1831 the Swedish researcher Sefstrom reidentified vanadium. Its discovery occurred when analyzing the iron extracted from the mineral obtained from the mine of Taberg. A student of the prestigious Swedish researcher Sefstrom Berzelius, he was responsible for spreading the results of the young Sefstrom internationally. The name of Vanadius was also put by the Swedes, based on the Swedish goddess of beauty and fertility ‘Vanadis’.

Shortly afterwards, German researcher Wöhler (and former student of the Berzeilus laboratory in Stockholm) stated that the eritronium and vanadium were the same element. For this purpose he used samples collected by Humboldt 30 years earlier. However, another 30 years passed until the English chemist Sir Henry Roscoe managed to isolate vanadium.
All these incidences make, although for some the discoverer of the vanadium is of the River, in some publications only the results of Sefstrom are mentioned. However, in most of the references it is considered the year 1801 as the date of the discovery of vanadium. Currently, an important Mexican research prize is named Del Río, and the Rio and Fausto Elhuyar are considered creators of Mexican science and research.
Mines and production
The main vanadium mines are in Australia, China, Russia and the South African Republic. At this time it is anticipated that the economically useful reserves amounted to 63 million tons. Given that annual consumption is 40,000 tons, it can be said that in the coming years there will be no supply problems.
In Russia, China and the South African Republic, vanadium is found in mineral magnetite. Therefore, vanadium is obtained jointly with iron. Iron is obtained by reducing magnetite in high furnace or solid state, being in both cases 1.5% of the extracted iron. Vanadium leaves the iron like slag.
In addition to iron mines, there are other sources for obtaining vanadium. In the United States, for example (Colorado), vanadium is usually accompanied by mineral uranium, while in the Caribbean and Venezuela, oil.

In general, it can be said that vanadium is produced as oxide. This oxide is used in the production of ferrobanadio and vanadium-aluminum alloys, the most interesting products from the point of view of industrial use.
Vanadium production grew gradually. Throughout the twentieth century. At the beginning of the century the production was very small, and until 1920 the world production did not reach 200 tons. Two years later, production multiplied by ten, but until the beginning of World War II it did not exceed 5,000 tons.
On the other hand, in this decade the consumption has doubled, going from 20.400 tons in 1991 to 40,000 tons in 2001.
Applications of vanadium
The studies of Seftrom and Roscoe focused solely on the use of vanadium in chemical components, which meant their first application. Until 1900 the vanadium was very expensive and quite curious, so its application was reduced. Later, XX. At the beginning of the twentieth century, after finding large amounts of vanadium in some mines, its price was significantly reduced and began to be used industrially.

In those years it was discovered in Germany that vanadium salts could be used as catalysts in some chemical reactions, this being the first important industrial application of vanadium. Catalysis remains the main chemical application of vanadium. At that time, the metallurgical process of vanadium was well advanced and different types of ferroalloys began to be produced commercially. Thus, in Wales was built the first productive unit that used vanadium as an alloy element in steels. In addition, Professor Arnold of Sheffield was requested to analyze the influence of vanadium in different types of steel. Arnold realized that vanadium was a very appropriate element to increase the resistance of tool steels, delaying the softening effect that these materials suffer at high temperature. These features make vanadium one of the components of cutting tools.
As a result of these and other investigations, England and France began to incorporate vanadium into the steel storks of cars to increase the resistance of crankshafts. And then came a chance that revolutionized the use and market of vanadium. Henry Ford discovered that in a car race held in Florida the chassis of a French car was different. Several pieces of steel of the car were smaller than the views so far and, in addition, three times more resistant than conventional ones. Ford himself directed the investigation into the characteristics of these steel pieces and found that the steel of the French racing cars had vanadium.
In the famous model T made by Ford, for the first time steel pieces were used alloyed by vanadium in a conventional car (and not for racing). And not just that. In the dismissal propaganda of the car the advantages of the car were attributed to vanadium. In fact, the announcement that appears in the first issue of October 1908 of Life magazine expressly mentions the extraordinary properties of ‘Ford Banadio steel’. Model T began to be manufactured in 1908 and until 1927 Henry Ford did not see the need to create a new model. During this period, 15 million cars of this model were sold. At that time, in the United States they did not know how to manufacture steel with vanadium and also took care of Henry Ford steel production.

The next great vanadium challenge should be related to steel improvements for structures. As a result of World War II, welding became the main binding procedure for Western countries. The technicians soon realized that not all the steels were able to weld (remember, if not, how many accidents and failures the American ships Liberty suffered during the war, which were loaded with cracks and defects by the welds).
For steel to be weldable, it was considered convenient to reduce the amount of carbon and adjust the chemical composition, losing resistance to steel. Several procedures were devised to produce weldable steel without loss of resistance, being one of the most important the manufacture of microalloys of steel and vanadium.
This type of steels are now called “high strength microalloyed steels”. The economic importance of these steels has increased year after year. Not all steel steels have vanadium, since niobium and titanium are also widely used in microalloys, but vanadium steels can be found anywhere.

As in the time of Henry Ford, many steel components of cars (starting with the crankshaft) still have vanadium. Also in the field of construction, an important method to increase the resistance of metal beams is based on vanadium. In fact, if you can increase the resistance of beams and pillars, these can be narrower, facilitating welding procedures and reducing construction costs. For these reasons, the use of vanadium is increasing.
As can be seen, the increase in steel resistance is an urgent goal and, with the use of vanadium, can be made easy and cheap. In many steels of different quality vanadium is an important additive, since adding small amounts of vanadium considerably increases the resistance of steel. By weight, the amount of vanadium is usually 0.1% and is very rare to contain more than 0.2% by weight. All these products are produced in various locations in the Basque Country.
These advantages make vanadium mainly used in the steel sector, which means 85% of the vanadium consumed. In the case of steels, their use is mainly focused on microalloyed steels for structures (65%), the rest is consumed in tool steels, high temperature steels, etc. The consumption of vanadium and the production of steel are therefore closely related. In the last decade an average of 0.05 kg of vanadium per ton of steel is used.
In aeronautics, vanadium is also widely used, in titanium alloys, to increase the resistance of the alloy.
Looking to the future, there does not seem to be major short-term changes, so it can be said that vanadium will be used primarily to increase steel resistance. However, in view of the results of some industrial investigations and tests, it seems possible to open new doors. The use of vanadium pentoxide for the production of batteries allows, successfully, to drastically modify the vanadium market.
Economic outlook Economic outlook
Asking the head of any Basque bakery about vanadium, he will immediately answer that the main problem of vanadium is the cost. And so it is. In fact, considering that thousands of tons of steel are produced every year in the steel mills, although small amounts of vanadium are added, it is expensive.
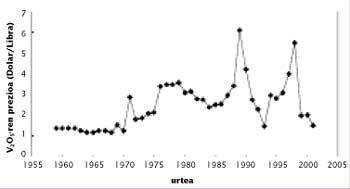
One of the characteristics of the price of vanadium is its variability. To understand this, we must take into account the imbalance between production and consumption. Given the low world consumption of vanadium, the system is unbalanced with relative ease in the commercialization of stocks or in the start-up of new mines, which translates into an important imbalance. For example, in the 1970s new mines were discovered in China and the South African Republic, resulting in overproduction. In the next two decades, the Soviet Union marketed its stocks at a price lower than that of the market, which reduced prices below 3 dollars.
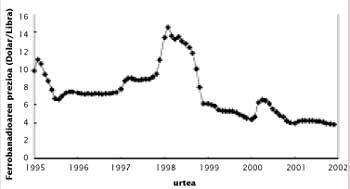
At the end of the 1980s and following, the increase in steel production led to an increase in consumption and a decrease in reserves in Russia, South Africa and the United States. In 1997 there was a quite serious situation, since the consumption of vanadium pentoxide reached 80,000 tons, which meant the emptying of stocks of almost everyone. Think that the U.S. government launched the vanadium in the National Defense Stockpile. In the coming months the price of vanadium was sold more expensive than ever, but since then the price has decreased considerably due to the reduction of consumption in Russia.
Depending on the use to which it is used, vanadium is sold as pentoxide and ferrobanadium. Therefore, in the market, the prices of these two products and not only of the distribution are used. The South African Republic is the one that produces more vanadium pentoxide, with almost 60% of commercialization. The production cost of the pound is around 1,8-2 dollars.
Currently the base price of the vanadium is $ 2,5. When the low price of this limit, some manufacturers cut the production until the price returns to its minimum value. As can be seen in the figure, in recent months the pentoxide is very cheap and the price of ferrobanadium also decays. Low prices will encourage the consumption of vanadium. However, in view of the upward trend shown by the production of microalloyed steels, even though prices rise to more normal values, consumption is expected to grow slowly in the coming years.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia






