Special hearts, special attention
2024/09/02 Aramburu Montenegro, Jorge - Ingeniaritza Aplikatuan doktorea eta TECNUN - Nafarroako Unibertsitateko irakasle eta ikertzailea Iturria: Elhuyar aldizkaria
Our body is a very complete and efficient machine; the heart, the engine of this machine. Sometimes, however, the motor comes from the factory with some peculiarity, for example with some innate disease, as occurs in 1 in 100 children born [1]. This directly influences the functioning of the body. In this article I will refer you to the blood circulation of people born with a single functional ventricle, from the point of view of fluid mechanics.
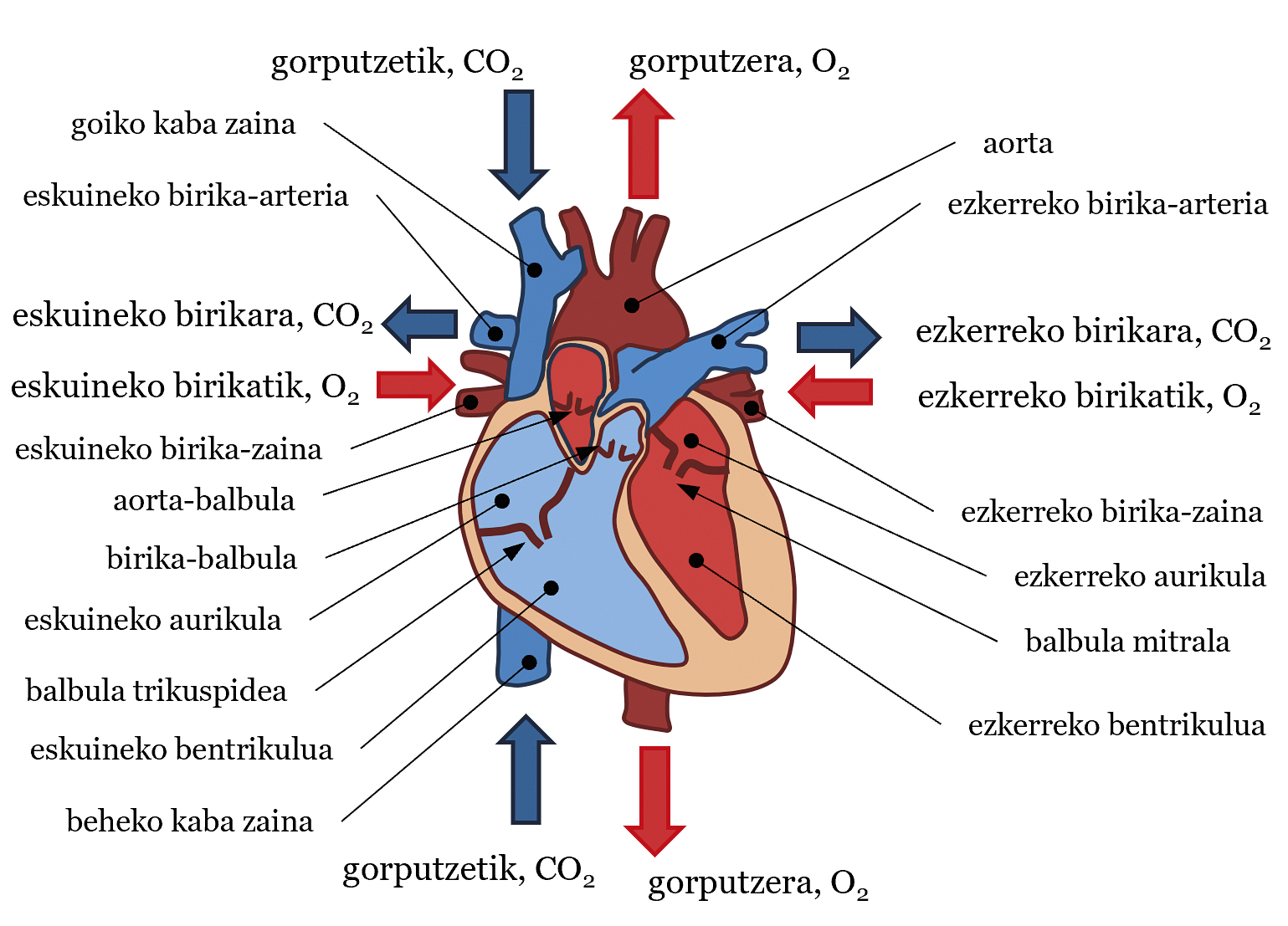
The cardiovascular system is a very complex structure consisting of three basic components: heart, blood and blood vessels. In general, the heart pumps blood and blood vessels transport blood to all parts of the body to perform various vital functions. These obligations include bringing food and oxygen to tissues, combating disease, etc. It is often said that the heart is our motor and, as if a water pump were to be treated, it pumps blood into the circulatory system. As for the structure, the heart can be divided into two parts, the left and the right, each with an atrium and a ventricle. Between atria and ventricles there are valves: mitral valve in the left heart and tricuspid valve in the right. An aortic valve in the left ventricle and a pulmonary valve in the right ventricle are also available at the exit of the ventricles (see Figure 1). Blood is also a very special fluid. 55% of its volume is fluid, plasma - almost everything is water - and the remaining 45% is made up of particles - almost all red blood cells. The direction of the flow or movement of this fluid, that is, the blood flow, is that which goes from high pressure areas to those of lower pressure. Finally, there are three types of blood vessels: arteries, veins and capillaries. The arteries carry blood from the heart to the body, the veins from the body to the heart, and in the capillaries, food and gas are exchanged between the blood and the tissues. By joining the latter to the idea of pressure, in general the blood has more pressure in the arteries than in the veins.
In this system, the heartbeat sets the rhythm of blood flow. Simply, we can describe the trajectory of blood flow in each beat (see Figure 1). Ventricular contraction increases the blood pressure of the ventricles. When this pressure is higher than that of the aorta and the pulmonary arteries, the aortic valve and the pulmonary valve are opened, pumping blood into systemic and pulmonary traffic, respectively. In systemic traffic, blood is pumped with adequate saturation or amount of oxygen from the left ventricle to the aortic artery. From here it is distributed to all parts of the body until it reaches the capillaries. There, food and gas are exchanged between blood and tissues. Ultimately, the blood leaves oxygen in the tissues and stays with carbon dioxide. Then, the blood moves from the capillaries to the veins, from there to the superior and inferior vena cava (SVC and CVI) and, finally, to the right atrium. In the pulmonary circulation, the blood that carries CO2 is channeled from the right ventricle to the left and right pulmonary arteries (LPA and RPA, left pulmonary artery and right pulmonary artery), from the arteries to the capillaries located in the pulmonary alveoli. In the Alveoli, blood will take O2 from the air and CO2 will be sent. Blood passes from the capillaries into the pulmonary veins and reaches the left atrium. The blood that has reached the right and left atria passes through the tricuspid valve and the mitral valve into the ventricles when the pressure of the atria is higher than that of the ventricles. Then, with the next beat, the blood flow path will resume, and so on. In this circulatory system, systemic circulation and pulmonary circulation are well differentiated, so blood with a large amount of O2 is well separated and with a large amount of CO2 (see Figure 1). Although in this article the fate of the blood has been explained, in each heart beat there are several phases that will not be explained here: isobolumetric contraction, ejection, etc. His explanation would be for another article.
Monoventricular patient and traffic in Fonta
We will then use the example of a patient born with hypoplasia of the left heart to explain the circulatory system of a person with a single functional ventricle (see Figure 2(a)). In this case, the left ventricle has not developed enough and cannot pump blood. Consequently, the right ventricle should pump blood, both into the pulmonary and systemic circulation, thanks to the connection between the pulmonary arteries and the aorta. In addition, the wall between the atria is usually perforated, so the systemic circulation and the pulmonary circulation are not well separated, as the blood of one and the other is confused (see Figure 2(a)). Thus, oxygen is not properly distributed throughout the body [2].
In order to improve the condition of these patients, Fontan and Baudet designed an operation in 1968 to separate the systemic circulation from the pulmonary circulation [3]. In it, surgeons, as if they were plumbers, create a cross structure with blood vessels. In fact, the superior and inferior vena cava join the left and right pulmonary arteries, building what is known as total cavopulmonary connection or CPC. They also attach the aorta to the ventricle (see Figure 2 (b)). This new circulation system is known as traffic name in Fontan.
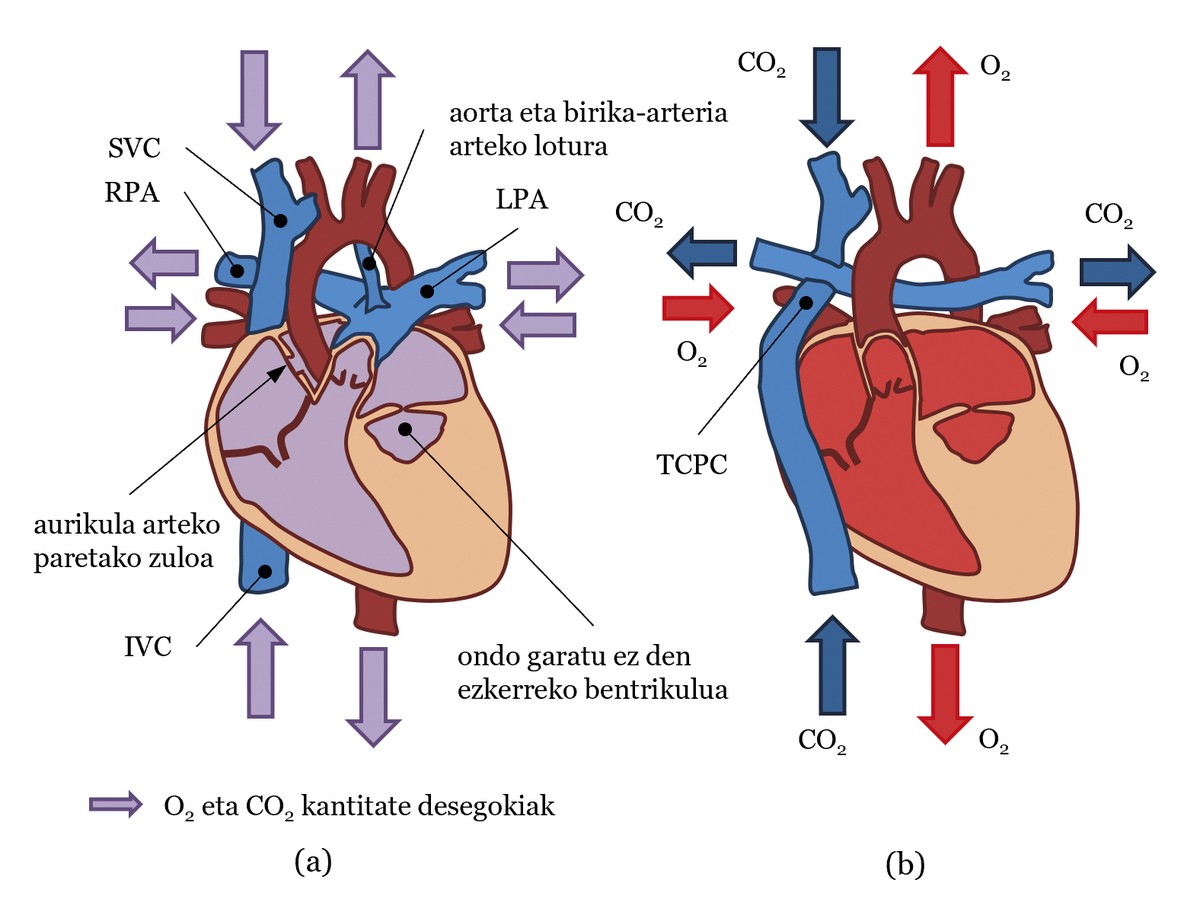
In the circulation of the Fountain, the ventricle working in each beat pumps blood to the aorta with the appropriate amount of O2 to reach the body tissues for the O2/CO2 exchange. This blood will arrive through the veins to the CSCT and through the vena cava it will be directed to the pulmonary arteries without passing through the heart. Thus, the blood carrying CO2 will be transferred to the lungs and the exchange of CO2/O2 will occur in the lungs. Finally, blood with the appropriate amount of O2 reaches the heart through the pulmonary veins. In the heart, blood moves from the atrium to the ventricle and the ventricle pumps to the aorta. As can be seen, systemic traffic is separated from the lungs. Another important point is that in the circulation of Fontan the work of the two must be performed by a single ventricle, since the blood must pass through the systemic circulation and the pulmonary circulation, which considerably increases the work of the ventricle.
Improving the quality of life of patients in Fontan
After the intervention, and only then, it is necessary to analyze the proper functioning of the circulation in the Fontana by analyzing several metrics. In any case, today it is possible to predict, in part, how the circulation in the Fountain will be. One of the ways to do this is by computer blood flow simulations or CFD simulations [4]. In fact, an expert in fluid dynamics, for example, a mechanical engineer, would be able to design the optimal CPC in collaboration with surgeons. Thus, the surgeon would be obliged to perform an intervention compatible with this design and to ensure the correct circulatory system of the patient.
Below is an example using CFD simulations. To do so, we will analyze two models. One, the model based on the CPCT of a real patient (hereinafter, model A). The other, like model A, but with the left pulmonary artery at 27 mm. below (hereinafter Model B) (see Figure 3 (a))). The simulations will be performed from the measured blood flow (ml/s) and pressure (mmHg) in the actual patient and the results will be analysed according to two characteristics. On the one hand, the loss of energy from blood flow in the HSCT. As for the loss of energy, let us remember that a single ventricle does the work and that the CPCT is a structure created by the surgeon; if the junction is not performed correctly, a significant loss of energy can occur. On the other hand, the distribution of blood flow in the pulmonary arteries. It is important that blood flow is evenly distributed between the left and right pulmonary arteries, about 50% each. It is also important to balance the blood flows from the superior and inferior vena cava in the two pulmonary arteries. For example, it is important that approximately 50% of the left pulmonary artery comes from the inferior vena cava and the other half is superior [4].
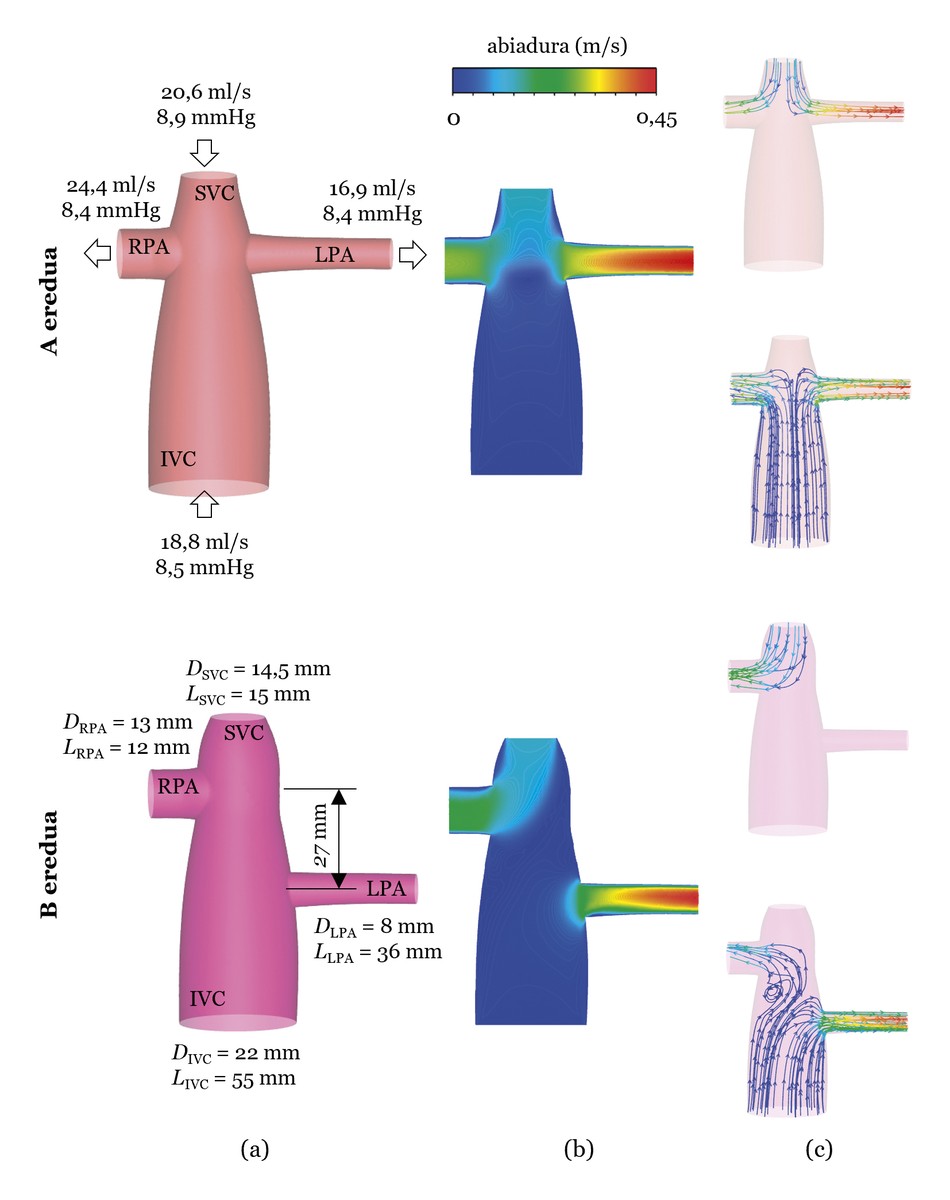
The simulations results can be seen in Figure 3 (b and c). Regarding energy loss, model A shows a 2.6% question and model B 2.2% question. Model B seems, therefore, better, but if the distribution of blood flows through the vena cava is analyzed, it is observed that in model B all the blood flow circulating in the superior vena cava is directed to the right pulmonary artery, so the blood circulating in the superior vena cava does not go to the left lung, which can cause problems. Thus, model A is more appropriate, although the loss of energy is higher than in model B, where the loss of energy is 2.6%, although it can be considered very low.
This shows that the geometry of the CPC generated by the surgeon influences the patient's circulation system and that computer CFD simulations can be useful for the design of the optimal CPC. However, there are numerous disciplines that combine engineering and medicine through CFD simulations. For example, in an earlier article it was explained that in a treatment against liver cancer, in radioembolization, the work of engineers could be important [5].
Finally, it must be stressed that interdisciplinarity, in general, is very important and necessary for progress to be made in all areas. Because interdisciplinarity is plurality and diversity is always wealth.
References
[1] Liu Y. Chen S., Zühlke L., Black G. C., Choy M., Li N. and Keavney B. "Wow, sir!" 2019. “Global birth prevalence of congenital heart defects–2017: updated systems ematic review and meta-analysis of 260 studies”. International Journal of Epidemiology, 48, 455–463.
[2] Barron D. J., Kilby M. D., Davies B., Wright, Dr. G. C., Jones T. J and Brawn W. J. 2009. Hypoplastic left heart syndrome. Lancet, 374, 551-564.
[3] Fontan F. and Baudet E. 1971. “Surgical repair of tricuspid atresia”. Thorax, 26, 240-248.
4] Slesnick T. The great C. 2017. “Role of computational modelling in planning and executing interventional procedures for congenital heart disease”. Canadian Journal of Cardiology 33, 1159-1170.
[5] Aramburu J. 2018. “Joining forces in the fight against liver cancer.” Elhuyar, 329, 68-72.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia