CO2 et al.
2009/06/01 Kortabitarte Egiguren, Irati - Elhuyar Zientzia Iturria: Elhuyar aldizkaria

The Earth's atmosphere consists of several gases. The main gases are nitrogen (78%) and oxygen (21%). These six greenhouse gases are found in small amounts: CO 2, 378 ppm and CH 4 to 1.774 ppb. That is, if you take a liter of atmosphere and divide it into a million, CO 2 would divide into 378 parts, while methane would only reach 1.774 of every billion parts. They therefore occupy a very small part of the atmosphere. However, although they manifest in very low concentrations from the volumetric point of view, they have a great influence on the greenhouse effect. In fact, greenhouse gases absorb the infrared rays emitted by the earth's surface and do not let them escape. In this way, the Earth is warming. And among the greenhouse gases, CO 2 is the king of all.
Calorific power
To understand why there is so much talk about CO 2 you have to understand how the influence of each gas is measured. Predicting the influence of each gas is no easy task. Meteorologists generally use two concepts: global warming potential (GWP) and radiative forcing. Global warming potential is an index that represents the influence of a substance on global warming. This index is calculated based on the warming produced by the amount of carbon dioxide in the same mass (CO 2 is assigned the value 1). It represents the relative importance of greenhouse gases to CO 2 over a given period of time. In a given period of time, not all gases remain the same in the atmosphere. Thus, the calorific power depends on the gas's ability to absorb infrared radiation and the time remaining in the atmosphere. For example, over a 20-year period, the warming that a kilogram of methane can produce amounts to 62 kilograms of carbon dioxide, while over a 100-year period it amounts to 21-23 kilograms of carbon dioxide.
However, in addition to global warming capacity, the amount of these gases in the atmosphere must be taken into account. In fact, the heating potential of methane is 21 times higher than that of carbon dioxide, taking as reference the period of 100 years. However, considering that the concentration of CO 2 is much higher than that of methane, it is observed that the effect of methane on climate change is actually less than that of CO 2. Let's not say in the case of SF 6. It has a GWP 22,000 times higher than CO 2, but hardly exists. Therefore, its incidence is very low compared to CO 2. In general, it is the case of fluorinated gases (HFC, CFC and SF 6). The total GWP of these gases is high and their concentration is low. However, gases remain in the atmosphere for a long time.
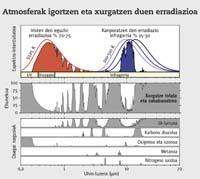
Therefore, radiation action is a more coherent unit than global warming capacity. In fact, this unit, in addition to the heating power of each gas, takes into account the concentration of each of them and the fluctuations that have occurred over the years. Thus, each gas is assigned a value. If this value is positive, it is often said that these gas molecules tend to heat the terrestrial surface, and if it is negative, to cool down. Of course, all the gases mentioned above are positive. In short, the radiation action measures the gross variation of the tropopause energy flow. That is, it measures the imbalance between the entrance to the atmosphere and the exit of the atmosphere through the W/m 2 unit. These imbalances may be due, among others, to changes in the concentration of greenhouse gases.
Given all these factors, no one questions that CO 2 prevails in all of them. CO 2 can last thousands of years in the atmosphere, and in 100 years it produces only a quarter of the impact this gas can cause.
"The concentration of CO 2 has grown spectacularly in recent years and, although the increase in this gas has stagnated, the global temperature would increase as the current climate is not balanced. In this sense, all models agree," explains UPV physicist Jon Saenz.
The "debate" of water vapor
In addition to the six greenhouse gases recognized by the Kyoto Protocol, there are others, such as water vapor, which some experts consider the main cause of the greenhouse effect.
For Saenz this question of water is an excuse. "To override the importance of CO 2, several arguments are sometimes used. This is the case of water vapor. Water vapor is often said to have a higher greenhouse effect than CO 2. The truth is that water vapor lasts very little in the atmosphere, 9 or 10 days, according to estimates. This means that a molecule of evaporated water in Mongolia travels several kilometers in the atmosphere and then disappears by precipitation," says Saenz.

"With the installation of one billion thermal power plants and the consequent evaporation of water, the average concentration of water in the atmosphere does not vary. Depending on the temperature, the water concentration is controlled by the Clausius-Clapeyron equation. If the temperature increases, more water accumulates in the atmosphere, so there will be more water vapor. However, if a water molecule, or ten thousand molecules, or ten billion surplus molecules, what will happen? It rains for ten days and with it water disappears from the atmosphere. That is, in ten days it disappears. When we talk about climate we talk about periods of one hundred years. The appearance of a ten-day imbalance in that time interval compensates. The GWP of water vapor is not calculated because its duration is very reduced on our planet and the concentration remains approximately constant, so the radiation of water vapor is not taken into account", added Saenz.
Therefore, the debate on greenhouse gases focuses mainly on long-term gases in the atmosphere, being the most important CO 2, CH 4, N 2 O and fluoridated. In general, 97% of the greenhouse effect causes it. Therefore, it can be said that they are the main responsible for the greenhouse effect.


Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia





