First treatment for Creutzfeldt-Jakob syndrome
2001/08/16 Astobiza, Amaia
B. Stanley The research team led by biochemist Prusiner has been experimenting with mice and has found that to date malaria and lambliasis, on the one hand, and schizophrenia and other psychotic diseases, on the other, two drugs used to treat can be used in the fight against Creutzfeldt-Jakob disease. Both drugs are quinacrine and chlorpromazine, respectively.
Creutzfeldt-Jakob disease is one of the variants of human spongiform encephalopathy. Spongiform encephalopathy is caused by prions, infectious particles formed by mutant forms of the Prp-c protein that appears in all living beings. In natural conditions, these proteins do not cause damage, while mutant forms are located on the neuronal surface producing degenerative diseases of the nervous system.

This is because the spiral structures of infected proteins expand and, in addition, they are associated with adjacent proteins and cause the expansion of their structures. Diseases resulting from this change include mad cow disease, scrapie (sheep and goat) and Creutzfeldt-Jakob syndrome. Especially infectious prions may appear by inherited genetic mutation and transmission.
First with the mouse, then with the man
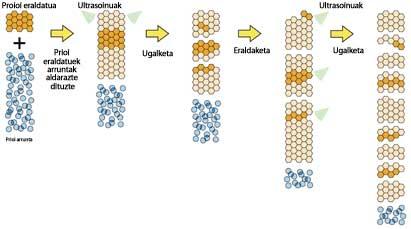
The two drugs selected by the researchers present a fundamental characteristic for fighting prions: they can cross the barrier between blood flow and neurons. Healthy Prp-cproteins are prevented from becoming infectious prions from quinacrine and chlorpromazine.
Mouse cells infected with prions were used to carry out the research. First, infected cells were treated with the drug. Subsequently, to ensure that the prions disappeared from the cells, treatment was interrupted and they saw that after three weeks no prions appeared. According to the researchers, these three weeks are necessary to say that the disease is actually cured.
Thanks to the good results obtained with mice, researchers want to see the influence of treatment on humans. So far two cases have been dealt with. One of them, 20-year-old English Rachel Forbes, was given a combination of two medications and saw that in 19 days his situation improved greatly. However, the other patient did not improve. Therefore, researchers believe that we must be cautious. In the coming months, a greater number of trials are planned to analyze and determine the usefulness of each drug separately and its combination in humans.
In addition, researchers do not know very well what mechanisms drugs use to remove prions from cells. Experiments have shown that quinacrine and chlorpromazine do not appear to be associated with prion to prevent the change from being transferred to other healthy Prp-c proteins. However, both drugs have a common characteristic of the structure: they have a tricyclic structure (of three rings), of which a lateral chain formed by molecules is central. Apparently, this lateral chain is essential for the efficacy of the drug.
More research
In future research, drugs, individually or in combination, will try to answer the question of whether they affect patients with advanced disease. The incubation of prion disease requires three to forty years, but when the first symptoms appear, the disease develops rapidly and, in general, at 12 months the patient dies. All patients to be treated will be in the final phase of the disease and the doses to be given will be the same as those used to treat malaria and schizophrenia.
At best, it would be possible to remove prions and repair the damage caused to neurons. In this case, the disease would be completely cured or at least the patient would improve. Another option is that, despite the disappearance of prions, damage to neurons is irreparable. In this case, the drug may prevent the disease from progressing. However, death can already be inevitable. The last option is to have a prophylactic effect on those who still have no symptoms. Thus, treatment could be used in patients who have inherited the disease through a genetic mutation and who have become ill from contamination of prions.
Neurologists and biochemists work in the group of researchers. The research is led by biochemist Prusiner and neurologist Carsten Korth and its results were published in the journal Proceedings of the National Academy of Science, on August 14. In 1997, Prusiner won the Nobel Prize in Physiology and Medicine for discovering that degenerative nervous system disease, called spongiform encephalopathy, is caused by prions. He currently works at the University of California School of Medicine in San Francisco (USA).

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia