When Lavoisier lost his head
2002/05/01 Carton Virto, Eider - Elhuyar Zientzia Iturria: Elhuyar aldizkaria
Antoine Laurent Lavoisier was born in Paris on 26 August 1743. His father was a rich lawyer. At the age of five his mother and father died, his little sister and the three went to live at his grandmother's house. There they grew up in the shelter of their grandmother and young aunt.

Lavoisier's ingenuity was evident as a child. He won several awards at school, especially in the field of languages, and in the second year of school he was given a special prize for his passion for learning. But in 1760 his young sister died and a year later he left studies unfinished. According to his father, he began to study the laws and in 1764, after completing his studies, he dedicated himself to advocating. However, it lasted little. He then resumed science and in 1768 he was appointed a member of the French Academy of Sciences.
Scientist with method
Lavoisier applied mathematics and the scientific method to research and denied several theories in force at the time, such as the ‘flogist theory’, which was definitively discarded by Lavoisier. To explain the combustion of bodies and the calcination of metals, this theory proposed the loss of flogisto of bodies.
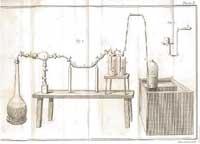
Lavoisier did not accept the theory and began to heat several substances in the air. Thus, he verified that the diamond was composed mainly of carbon and that, in the absence of air, the diamond was not burned. He also burned phosphorus and sulfur, and found that the products obtained weighed more than the original ones. Therefore, he considered that when burned some substance was added from the air, since he did not believe that the flogist could have a negative weight.
To demonstrate his idea, he heated tin and lead in a limited amount of air. The oxide layer appeared on the metals and was heavier than the metal itself. But Lavoisier proved that the whole of metal, its rust and air did not suffer any weight variation. Therefore, the weight that gained the rust would probably take off into the air. If the air was removed, the vacuum would be inside the closed container. As Lavoisier thought, when opening the container the air entered the interior: the rust was due to the combination between metal and air and there were no losses of flogisto. The theory of flogisto declined. Lavoisier showed that matter is not produced or undone, but changes state thanks to chemical processes.
Lavoisier demonstrated that metals are combined with air gas and oxides form. According to him, there were two gases in the air: one fired and the other did not. He called oxygen to the first (in Greek it means acid generator because he believed that all the acids had it) and to the second, azoe (it means not alive in Greek, later it was called nitrogen).

In addition to numerous studies, he made important contributions to the method and the systematic. As soon as he began his chemical investigations, he realized the importance of the precision of the measurements and the need to weigh reagents and products in any reaction, developed methods of analysis... in short, laid the foundations of modern chemistry. He also worried about nomenclature and in 1787, with the help of Berthollet and Forcroy, published the book Methods for Chemical Nomenclature. In 1789 he published his new doctrine in the book Traité élémentaire de chimie. It was the first modern chemistry textbook. In 1790 he assumed the responsibility of adding the units of weight and measure, and a year later the proposal to take the decimal system went ahead.
Economist Lavoisier
Antoine Lavoisier also did important political and economic work. In 1768 he began working as a tax collector in the company called Ferme Generale, and soon became a shareholder of the company. This company had an agreement with the French government to collect taxes and keep as a winner at a higher level. In 1771 he married the 14-year-old daughter of a companion who, with light and great gift of relationship, provided Lavoisier with invaluable help.
Lavoisier was responsible for the collection of salt, tobacco and alcohol taxes, and although he promoted concrete industrial policies and modernized factories, he gained a reputation. In fact, it implemented very strict measures in the manufacture of tobacco powder and sold rot tobacco. Also, since he was in charge of collecting taxes on goods entering Paris, he surrounded the city with a wall to avoid smuggling, but people did not like it.
Lavoisier also took care of the welfare of society. In 1760 he developed a system to improve the lighting of the villages and was a pioneer in the field of public health.
In 1780 the journalist Jean Paul Marat asked him for his entry into the Academy of Science and Lavoisier advocated refusing because his works had no scientific value. When the revolution arose, Lavoisier was imprisoned. In the trial Jean Paul Marate made him a harsh accusation of revenge for the problem of the Academy of Sciences. Although Marat died in July 1793, Lavoisier, his father-in-law and other members of Ferme General were guillotined on May 8, 1794.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia






