Metallic plastics Metallic plastics
2003/03/01 Arrillaga Laka, Alexander | Egurrola Agirre, Jose Javier | Zaldua, Ane Miren Iturria: Elhuyar aldizkaria
In recent years, many methods of polymer metalization have been used, such as vacuum metallization or cathodic spraying ( Sputtering ), but lately the galvanic metallization is being imposed as one of the few processes of adaptation to the demanding demands of customers.
Galvanic metalization is based on an electrochemical process in which electrical energy is used to conduct a chemical reaction, that is, the reverse of what happens in batteries. In galvanic metalization, the metallising piece acts as negatively charged electrode. The piece attracts and reduces dissolved metal cations (copper, chrome, etc. ), leaving the piece covered by a thin metallic layer.
But the main problem in the world of polymers is not this electrochemical process, but polymers in general are insulating. Polymers are not able to transport electricity and for its metallization it is necessary to convert the surface of the polymer parts into conductor. But how?
The suppliers of galvanic baths of polymeric materials have treated and continue to work on finding solutions to this problem. For example, paintings with small dispersed metal particles have been invented. The paint is applied on polymeric parts and these metal particles allow the electrochemical process. Another solution is the development of conveyor polymers.
The success of the metallization depends on the degree of usefulness of the piece obtained, for which a good polymer/metallic layer ratio is essential.
This is the main problem of the two techniques mentioned. Between the metal layer and the polymer surface, weak bonding forces are generated and in a short time the metal layer is separated from the piece.
Quality connection
Galvanic metallization is the most appropriate method for good adhesion of polymer and metallic layer. In the industry, mainly, we proceed to the metallization of polymers called ABS and ABS/PC through this process, being most automotive parts. With the polymer ABS antiradiant nets, brand symbols, etc.
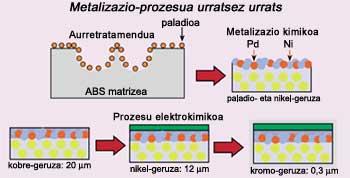
The process is divided into three steps. In the first place the pretreatment is carried out, that is, the surface of the polymer, in this case the ABS, is attacked chemically. ABS consists of three monomers, acrylonitrile, butadiene and styrene, but butadiene does not mix well with the other two and is dispersed. ABS is, therefore, a mixture in two phases, polybutadiene on one side and copolymer acrylonitrile/styrene on the other.
In this first step, by using strong acids, the existing butadiene is attacked on the surface, degrades and holes are created in the space where there was butadiene before. Then this new surface is covered with palladium particles.

Once the pretreatment is finished, the chemical metalization of the piece is done. The objective is to convert the polymer surface into a conductor through a chemical process that is covered with a thin metallic layer. The palladium added in the previous step serves as a catalyst and covers the piece with a thin nickel layer.
The electrochemical process ends. In this step three metal layers of copper, nickel and chromium are added to the ABS.
The result is a piece of plastic with a metal shape, but lighter and cheaper than that which would be a metal piece.
However, there are in the market parts that need to be metallized but that cannot be manufactured with ABS. Therefore, suppliers are developing baths for the metalization of polyamide and polypropylene and ABS with an unconventional composition, since these materials present better properties for some applications.
Currently there are two solutions for the metallization of parts that cannot be manufactured only with ABS: the part of the piece that will be metallised with ABS and the rest with another more appropriate polymer; or the surface of the piece with ABS and the core with others. In
spite of this, while the pieces that are intended to chromar can be manufactured by ABS, it seems that the metallising systems of other polymers will not develop in excess and that all the investigations and findings made will remain intact. However, in the ABS process there is still much to learn and analyze.
 (Photos: J.J. Wood).
(Photos: J.J. Wood).

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia