Placebos and ethics on both sides of the scale
2010/09/01 Galarraga Aiestaran, Ana - Elhuyar Zientzia Iturria: Elhuyar aldizkaria
Two years ago, a U.S. consultation was conducted to find out if doctors prescribed placebo to patients. The result was a stir, as of those who responded, about half recognized that they were receiving placebos, and only 12% totally rejected this behavior.
In Europe this research has not been carried out, so there is no data here. The US questionnaire was conducted by the Department of Bioethics of the Institute of Health. 1,200 internal medicine and rheumatology specialists participated, the results of which were published in the prestigious British Medical Journal. According to the data obtained, 57% responded and, of them, 62% thought that the prescription of placebos was "ethically acceptable".
Thus, according to the way in which the question was raised, between 46 and 58% recognized having prescribed during that same year a substance in the form of placebo, mainly non-prescription painkillers (41%) and vitamins (38%). A few ordered antibiotics or sedatives (13% in both cases) and fewer still used real placebos such as saline (3%) and sugar pills (2%).
In the opinion of supporters of the use of placebos, the placebo effect can be one of the most effective treatments for many chronic diseases and can be given without fraud. However, in the conclusions of the article, the authors noted that "doctors may not be completely transparent to their patients" and that they had confused reasons to prescribe these treatments, for example, that they felt "need to do something" to relieve the pain of patients, even though it was not proven to be effective. In the end, the authors claimed the need to promote a deep ethical and political debate.
The article received many answers. In the United States, the article written by ethical expert Antonella Surbone of the Department of Medicine at the University of New York was very influential. In that article he clearly expressed his opinion: if it is a placebo, the patient should be informed. In addition, Surbon considered that the placebo recipe to prevent the patient from feeling disappointed favors the culture of medicalization. That is, it reinforces the belief that everything can be solved with medicines.
Surbón finished his article with a suggestion in which he proposed to strengthen ties between patient and doctor convinced that the therapeutic relationship is based on truth and mutual trust. "It is not a placebo effect, but a therapeutic capacity for relationship, compassion and care."
Recyclable and not

Although this type of consultation has not been carried out in Europe, the doctor Iciar Alfonso, one of the founders of the Ethical Committee for Clinical Research of the Basque Country, has warned that in usual clinical practice it is not possible to prescribe a pure placebo, since among products susceptible to being prescribed there is nothing that contains exclusively ineffective substances.
Dr. Mikel Latorre reaffirms what Alfonso said. Latorre is a doctor at Cruces Hospital, has been working for years in the healthcare quality unit of patients and is a member of the ethics committee for clinical research. In his opinion, the pressure that patients exert on doctors is "increasing", so that, above the desire that doctors feel to help patients, this can be one of the causes that some doctors prescribe placebos.
However, he explains that the public health system does not allow the prescription of placebos: "I can not prescribe vitamins to a patient because they are not among the products that can be recipes, if the patient wants it can buy them, but without recipes. However, in the private health system it is possible to prescribe it."
Latorre, however, does not in any case consider it acceptable to order placebos. It coincides with the perception that prescription of placebos favors medicalization and the importance of working the relationship with the patient: "It is true that you feel pressure from the patient, but the problem that is generated has nothing to do with placebo, it is a relationship problem. The key is precisely to work on this relationship."
Placebos in clinical research
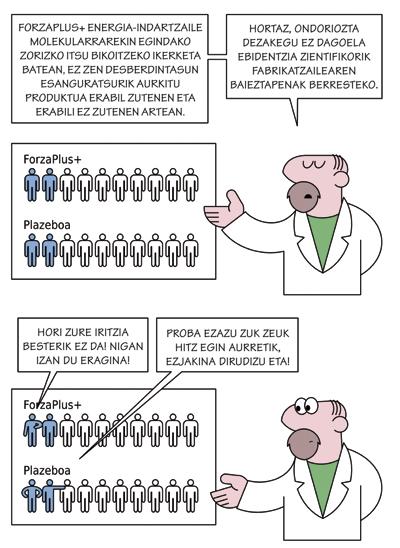
Pharmaceutical companies put even more pressure on clinical research than on medical consultations. According to Latorre, in the studies that the industry presents in the ethics committee of clinical research, they want to compare with placebo the medicine they want to try.
On the contrary, in the Cruces Hospital, they favor comparing the drug to be tested with the most appropriate treatment for this case, which is the most logical, according to Latorre: "The new medicine should show that it's better than there is, but of course, it's easier to compare your product to something that doesn't affect it and show that it has some influence. That's why they want to compare themselves to placebo, because if it's not more difficult to market new products."

In the words of Latorre, in Cruces they do not admit to comparing themselves with placebo, "if there is nothing else". In all other cases, they propose comparing them with the best treatment they have. In any case, the final decision is adopted by the Basque Country Commission.
The secretary of this committee is Iciar Alfonso, who has prepared a report that includes the ethical and methodological aspects of the use of placebo in clinical research. Among other things, the report literally captures Article 29 of the last revision of the Declaration of Helsinki. This statement, which includes ethical guidelines for those who experiment with people, states that "any new prophylactic, diagnostic or therapeutic method must be tested against the best current method, without prejudice to the use of placebo or the non-application of treatments in studies where there is no proven treatment."
Alfonso recalled that this article generated a "terrible debate" and in the end have had to soften this point in part. However, it is still valid, since the use of placebos in clinical research generates ethical problems. Some have been mentioned by Alfonso himself. On the one hand, giving placebo to the person involved in the research can be a "form of fraud"; "for example, patients can accept participation without understanding what it means to use placebo and what consequences it can have."
In addition, placebo can directly harm the research participant by refusing or delaying effective treatment. For Alfonso, this is the greatest ethical doubt generated by the use of placebo.
The ethical committees for clinical research are therefore concerned with these issues. Its main function is to ensure the rights, safety and well-being of people participating in clinical trials, evaluating the protocols of clinical trials presented to them, taking into account the methodological, ethical and legal aspect, as well as the balance between risk and benefit.
Thus, Alfonso explained that "for a group of patients to accept a placebo clinical trial, the potential risk of patients should be minimal. In addition, the reason for the use of placebo must be well analyzed."
However, he has recognized that sometimes the use of placebo in research has been beneficial and has mentioned the case of a medicine to treat arrhythmia: "In this case, the use of placebo was beneficial, which showed that the medicine that cured arrhythmia, according to the tests carried out until then, increased mortality." It is undeniable that placebo use has been key in many clinical studies.
Double ethical standard
However, the usefulness of placebos does not eliminate the ethical concerns it generates, which are aggravated when clinical trials are conducted in non-industrialized countries. In fact, the researcher of the Chair of Law and Human Genome, Leire Escajedo, has warned that in some cases they use the double standard. In other words, whoever directs and pays for research does not act in the same way, if the country in which the research is conducted is industrialized or not industrialized.
In fact, the Declaration of the Union of Bioethics and Human Rights does not recognize this behavior. The second point of Article 21 of the aforementioned declaration states: "The conduct of a research activity in one or more States (the host State or host States) in another way and whose source of funding is in another State would constitute an appropriate ethical examination of such activity in both the host State or hosts and the State in which the source of funding is located."
However, according to Escajedo, "unfortunately UNESCO does not have sufficient forces to prevent anything", and not all comply with the recommendation. Thus, in Africa they accept research that in no case would be accepted in Europe or the United States, for example, anti-AIDS treatments that are tested with the use of placebo. It is an extreme example of the damage placebos can cause, the darkest side of a topic with many edges.

Gai honi buruzko eduki gehiago
Elhuyarrek garatutako teknologia